Report Overview
France AI-Driven Hypothesis Generation Highlights
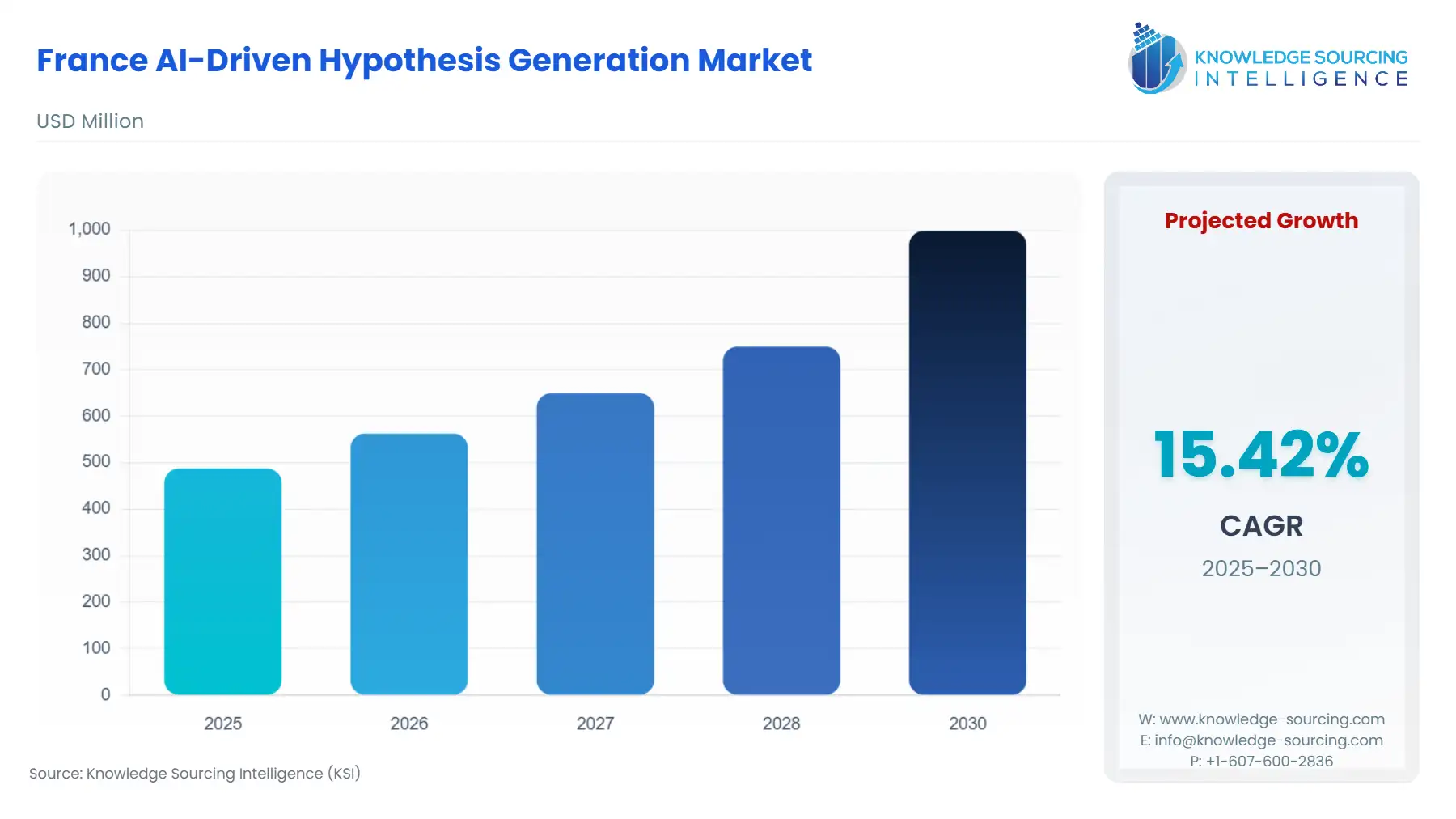
France AI-Driven Hypothesis Generation Market is projected to expand from USD 487.4766 million in 2025 to USD 998.63831 million by 2030, maintaining a CAGR of 15.42%.
France AI-Driven Hypothesis Generation Market Key Highlights
The French AI-Driven Hypothesis Generation Market, an integral component of the nation's ambitious "AI for Humanity" strategy, is evolving from an emergent technology sector into a foundational operational layer across high-stakes verticals. This market focuses on software and computational services that leverage advanced machine learning models, natural language processing (NLP), and knowledge graphs to analyze vast, disparate datasets (biomedical literature, patient records, financial market signals) and propose novel, statistically plausible hypotheses for experimental validation or strategic decision-making. The current market dynamic is characterized by the intersection of deep scientific mandates in the Life Sciences sector—France's stated priority—and complex European regulatory oversight.
France AI-Driven Hypothesis Generation Market Analysis
Growth Drivers
- France's dedicated effort to establish itself as an AI leader, exemplified by the EUR 1.5 billion committed by the French Government (EC AI Watch, 2021), serves as a fundamental catalyst. This public investment, particularly the portion directed toward research via the national research institute Inria and the 3IA network, directly increases the technical capacity and subsequent demand from academic and public research institutions for AI-Powered Literature Mining Tools that can process the resulting surge of open-source research data. Concurrently, the imperative within the private sector to accelerate drug discovery and minimize R&D expenditure directly propels demand for Domain-Specific Predictive Modeling Tools. For life sciences firms, integrating AI for de novo target identification or drug repurposing is no longer optional but a strategic necessity to shorten the time-to-market for therapeutic candidates.
Challenges and Opportunities
A critical challenge is the documented historical underperformance of France in the Biotechnologies domain (Belfer Center, 2025), including a reported lack of cooperation between universities and industry. This scarcity of collaborative, integrated data ecosystems constrains the real-world application of advanced AI models and lowers the effective demand from established biopharma for pre-trained systems. The primary opportunity lies in the stringent new European AI regulations. The necessity for traceability and human oversight in high-risk AI applications (e.g., medical devices, as per the EU AI Act) creates an immediate, distinct demand for Graph-Based Hypothesis Generation Platforms that offer transparent, auditable pathways from data input to hypothesis output, transforming a regulatory constraint into a core product requirement and competitive differentiator for domestic firms.
Supply Chain Analysis
The AI-driven hypothesis generation market relies not on a physical supply chain but on an Intellectual and Data Supply Chain. The primary input dependency is on high-quality, large-scale, annotated multimodal data—specifically, clinical and genomic data for the Life Sciences segment, which represents the largest demand vector in France. Key production hubs for the software itself are concentrated in major French tech ecosystems, namely Paris (Île-de-France region) and its surrounding hubs, where the majority of AI talent and venture capital is secured. Logistical complexities center on data governance and cross-border data federation, given that French AI firms often partner with European and US institutions (e.g., Owkin's collaborations across Europe and the US). The core dependency is on the national policy framework's success in incentivizing secure, federated data access models that adhere to GDPR, as opposed to centralized data repositories which would violate privacy laws.
Government Regulations
| Jurisdiction | Key Regulation / Agency | Market Impact Analysis |
|---|---|---|
| European Union | EU AI Act (Published July 2024) | Classifies AI in medical devices as "High Risk," mandating strict requirements for risk management, data governance, and high-quality training data. This regulation directly increases demand for auditable and explainable AI (XAI) platforms, favoring verifiable Graph-Based Hypothesis Generation over opaque "black box" deep learning models in critical applications. |
| France | "AI for Humanity" Strategy (Villani Report, 2018) | The explicit designation of Health as a priority sector, coupled with EUR 700 million allocated for research (EC AI Watch, 2021), generates sustained demand for all hypothesis generation tools (e.g., AI-Powered Literature Mining) across the French academic and hospital network (AP-HP, etc.) to accelerate translational research. |
| France / EU | General Data Protection Regulation (GDPR) | Restricts the central aggregation of patient-level data. This constraint catalyzes demand for privacy-preserving AI methods, such as Federated Learning, thereby increasing the demand for specialized, secure platforms (e.g., Owkin’s architecture) that can generate hypotheses without the direct transfer of raw patient data. |
In-Depth Segment Analysis
By Application Area: Drug Discovery & Life Sciences
The Drug Discovery & Life Sciences segment stands as the preeminent application area, driven by the unsustainable costs and failure rates inherent in traditional R&D. The need for AI-driven hypothesis generation here is explicitly linked to the need for a non-linear acceleration of preclinical research. The core growth signal is the industry imperative to reduce the $2.6 billion average cost and 10-15 year timeline of bringing a new drug to market. AI tools, particularly Domain-Specific Predictive Modeling Tools, are crucial for generating hypotheses regarding novel drug targets, predicting compound efficacy and toxicity, and identifying potential drug repurposing candidates from vast chemical and biological libraries. The verifiable success of French companies securing clinical partnerships (e.g., Owkin's extensive collaboration network) validates the demand for tools that can rapidly prioritize the most promising molecules and biological mechanisms, effectively shifting capital from high-risk, low-yield empirical experimentation toward high-confidence, in-silico hypothesis validation. This segment's growth is therefore directly proportional to the competitive pressure on French biopharma to shorten the discovery cycle.
By Technology: Graph-Based Hypothesis Generation Platforms
The Graph-Based Hypothesis Generation Platforms segment is experiencing a significant uplift, fundamentally driven by the dual mandates of scientific complexity and regulatory compliance. Scientifically, these platforms model biological or chemical knowledge as large, interconnected graphs (nodes representing entities like genes, proteins, diseases; edges representing relationships like "interacts with," "causes," or "treats"). The ability of these systems to traverse and combine disparate knowledge to propose novel relationships that a human researcher may overlook—such as a specific gene being implicated in two seemingly unrelated diseases—creates a powerful demand for deeper, systemic insights. Critically, these platforms inherently offer greater transparency than traditional deep learning models. By explicitly mapping the data pathways and logical rules that connect, for instance, a molecular entity to a disease outcome, they provide a necessary audit trail. This transparency is the direct answer to the traceability and explainability requirements mandated by the EU's High-Risk AI classifications, establishing Graph-Based Platforms as the preferred technology in regulated French clinical and diagnostic research environments.
Competitive Environment and Analysis
The French AI-Driven Hypothesis Generation Market is characterized by a mix of specialized domestic AI firms and traditional biotechs leveraging computational capabilities. The competitive edge belongs to those who successfully integrate access to protected clinical data with proprietary machine learning models.
Owkin
Owkin (Headquarters: Paris, France) is a leader in the field, strategically focused on federated learning to unlock siloed patient data across multiple hospitals without violating GDPR. Its strategic positioning is defined by an access-over-aggregation model. A key verifiable product is K Navigator, an agentic co-pilot designed to accelerate biomedical breakthroughs by enabling researchers to explore, refine, and validate hypotheses using a natural language interface, combined with exclusive access to proprietary data subsets like MOSAIC Window. This product targets the core pain points of data access and analytical complexity in research.
DBV Technologies
DBV Technologies (Headquarters: Châtillon, France) is a clinical-stage biopharmaceutical company. While not a pure-play AI vendor, its activities demonstrate the profound end-user reliance on hypothesis validation. The company’s focus on its Viaskin® proprietary technology for food allergies showcases a business model that generates substantial demand for hypothesis generation support, particularly in clinical trial design, patient stratification, and mechanistic hypothesis refinement. Its high R&D expenditure—$55.2 million for the first six months of 2025 (DBV H1 2025 Financials)—underscores the substantial investment being made in research, which drives internal and external demand for efficient computational tools to justify and guide those expenditures.
Recent Market Developments
- October 2025: DBV Technologies announced the sale of approximately $30 million of ADSs through its At-The-Market (ATM) Program on Nasdaq to Invus. This financing event, occurring at a clinical-stage company, represents a capacity addition/funding event that supports the continuation of its substantial R&D expenditure (which necessitates continued use of hypothesis generation tools) as it advances its Viaskin® Peanut patch.
- July 2025: Owkin announced a new five-year AI partnership with Newcastle upon Tyne Hospitals NHS Foundation Trust in the UK. This strategic agreement is a verifiable capacity addition, extending Owkin's federated learning network and its access to diverse clinical data, which is the foundational "raw material" for its hypothesis generation platform, accelerating drug discovery and diagnostics.
- May 2025: Owkin launched K Navigator, a "Ground-breaking Agentic Co-pilot to Speed up Breakthroughs in Biomedical Research by 20x." This verifiable product launch marks a significant technological advancement in the hypothesis generation market, introducing an agentic AI tool (K Pro to follow later in the year) that can analyze and visualize multimodal patient data and accelerate literature reviews across millions of scientific articles.
France AI-Driven Hypothesis Generation Market Segmentation
- BY SOFTWARE TYPE
- AI-Powered Literature Mining Tools
- Graph-Based Hypothesis Generation Platforms
- Domain-Specific Predictive Modeling Tools
- Multimodal AI Platforms
- Others
- BY APPLICATION AREA
- Drug Discovery & Life Sciences
- Healthcare & Diagnostics
- Materials & Chemical Research
- Financial & Business Analytics
- Academic
- BY DEPLOYMENT MODE
- Cloud-Based
- On-Premise