Report Overview
US AI-Driven Hypothesis Generation Highlights
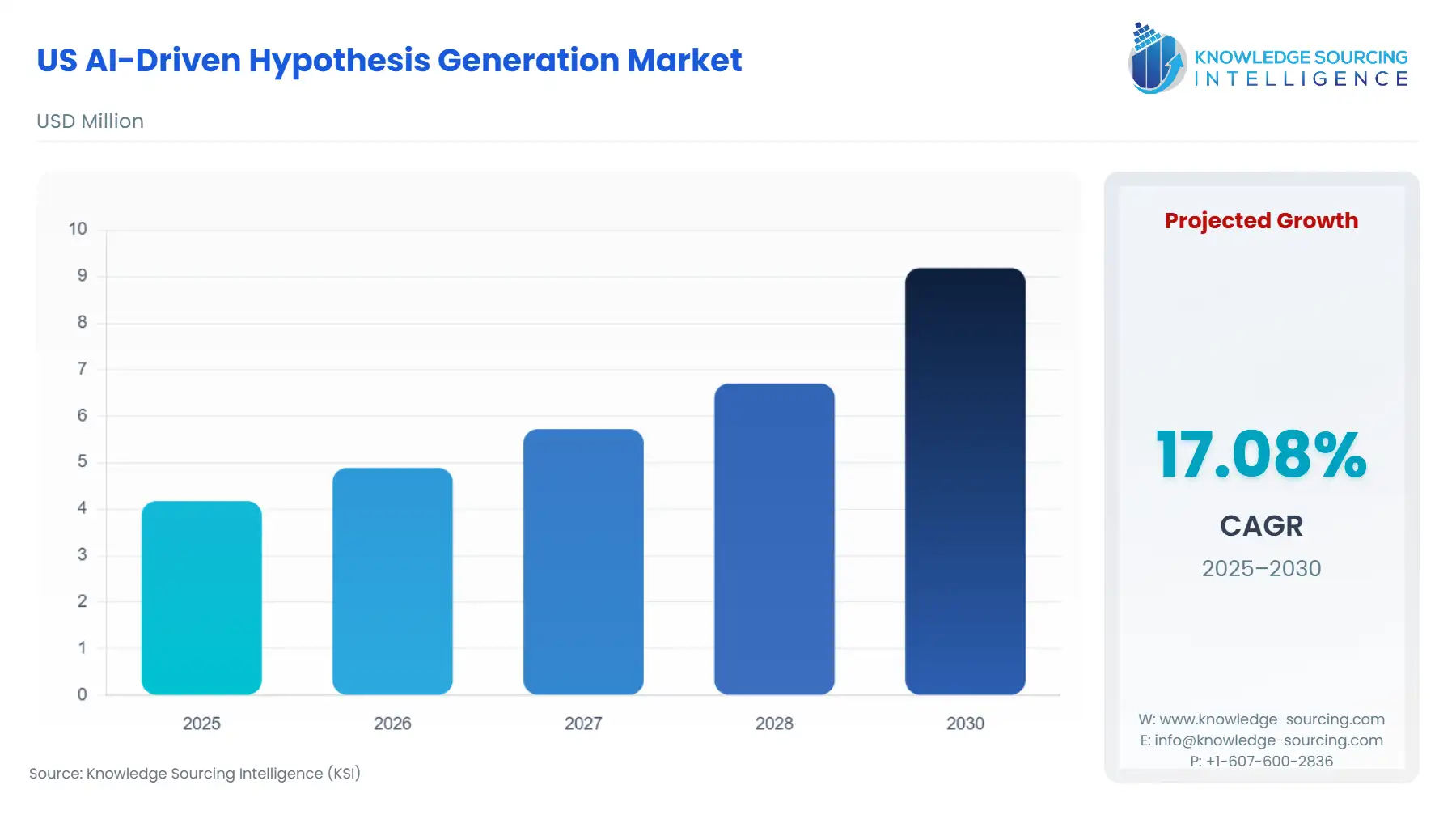
US AI-Driven Hypothesis Generation Market is anticipated to increase from USD 4.175712 million in 2025 to USD 9.1865664 million by 2030, with a CAGR of 17.08%.
US AI-Driven Hypothesis Generation Market Key Highlights
The US AI-Driven Hypothesis Generation Market is emerging as a foundational pillar within the broader data-intensive research ecosystem, specifically in life sciences and materials science. This market is characterized by software solutions that leverage machine learning (ML), natural language processing (NLP), and knowledge graph technologies to automate the formulation of testable scientific or business hypotheses from complex, unstructured, and disparate data sets. Historically constrained by the manual, time-intensive process of literature review and data correlation, the market is now experiencing rapid expansion. The value proposition centers on dramatically shortening the discovery cycle, reducing the high attrition rate of candidates in sectors like drug development, and uncovering novel, non-obvious insights that human experts frequently overlook.
________________________________________
US AI-Driven Hypothesis Generation Market Analysis
Growth Drivers
- Escalating Cost and Timelines of Drug Development: The escalating cost and protracted timelines of the drug development pipeline create a mandate for efficiency. The traditional 10 to 15-year drug development cycle, with a low single-digit probability of success, propels demand for AI tools that can de-risk and accelerate early-stage research. Specifically, the ability of AI to rapidly sift through chemical and biological data to predict molecular behavior and target-binding affinities increases the demand for Domain-Specific Predictive Modeling Tools that improve candidate selection. Concurrently, the proliferation of biomedical data, including petabytes of proprietary omics data generated by high-throughput laboratories and public repositories, exceeds human capacity for manual analysis. This data overload makes AI-Powered Literature Mining Tools and Multimodal AI Platforms an operational imperative for research institutions seeking to synthesize these disparate data streams into actionable hypotheses.
Challenges and Opportunities
- Data Harmonization and Quality: A primary constraint on market growth is the challenge of data harmonization and quality. AI-Driven Hypothesis Generation platforms are only as effective as their training data; thus, the industry's widespread adoption of siloed, poorly standardized legacy data infrastructure necessitates costly and time-consuming data preparation, which temporarily decreases the speed of software deployment.
- Graph-Based Hypothesis Generation Platforms: This challenge, however, concurrently creates a massive opportunity for providers of Graph-Based Hypothesis Generation Platforms that specialize in constructing harmonized knowledge graphs from heterogeneous data. This capability directly increases demand for platforms that can manage and query complex semantic relationships to reveal non-obvious links, transitioning a data challenge into a unique analytical opportunity.
Supply Chain Analysis
The supply chain for the US AI-Driven Hypothesis Generation Market is fundamentally non-physical, centered on talent, proprietary data, and computational infrastructure, with key dependencies globally distributed. The core production hubs are concentrated in US technology clusters, particularly California and Massachusetts, driven by access to deep-learning engineers and domain-specific scientists. The logistical complexities revolve around the secure, compliant, and real-time movement of vast patient and research data, rather than material goods. Dependencies include high-performance computing (HPC) resources, often provided by global cloud vendors (e.g., Google, Microsoft) whose data center capacity directly impacts the scalability and pricing of Cloud-Based deployment models. The most critical dependency is the continuous generation of novel, high-quality, fit-for-purpose biological and chemical datasets that serve as the "raw material" for model training and hypothesis validation.
Government Regulations
| Jurisdiction | Key Regulation / Agency | Market Impact Analysis |
|---|---|---|
| United States | FDA (Food and Drug Administration) Draft Guidance: Considerations for the Use of Artificial Intelligence to Support Regulatory Decision Making for Drug and Biological Products (January 2025) | Establishes a risk-based credibility assessment framework for evaluating AI models used in drug development submissions. This clarity reduces regulatory uncertainty for BioPharma companies, directly encouraging greater investment and adoption of AI-driven tools by defining an explicit pathway for their inclusion in the Investigational New Drug (IND) and New Drug Application (NDA) processes. |
| United States | White House Executive Order on the Safe, Secure, and Trustworthy Development and Use of AI (January 2025 Revised) | Mandates the development of standards for secure, transparent, and ethical AI, including in healthcare. This increases the demand for AI software with robust explainability (XAI) and auditability features, compelling vendors to invest in technologies that ensure model transparency to mitigate bias and liability risks in hypothesis formulation. |
| United States | Department of Health and Human Services (HHS) Strategy for Quality in AI-Enabled Healthcare Technology | Focuses on maintaining quality through premarket assessment and post-market oversight, requiring rigorous testing and validation standards for data quality and model reproducibility. This drives demand for vendors offering comprehensive data governance and validation services alongside their hypothesis generation platforms to meet heightened quality assurance requirements. |
________________________________________
In-Depth Segment Analysis
By Application Area: Drug Discovery & Life Sciences
The Drug Discovery & Life Sciences segment represents the largest and most critical application for AI-Driven Hypothesis Generation, as its demand is acutely driven by the industry's perpetual need to compress its R&D timelines and improve clinical success rates. The application of AI-Powered Literature Mining Tools is essential here, as the sheer volume of biomedical literature—millions of papers and patents—makes manual synthesis of potential drug-target-disease relationships untenable. Organizations require platforms that can automatically extract, normalize, and link entities (genes, compounds, pathways, diseases) across this corpus to generate novel hypotheses, such as predicting a secondary use for an existing drug (repurposing) or identifying a previously uncharacterized pathway. The validated success of AI-discovered drug candidates entering clinical trials acts as a significant growth catalyst, motivating competitive spending among pharmaceutical majors to acquire and integrate these platforms into their preclinical workflows. This directly addresses the high attrition rate by front-loading the de-risking process with data-driven insights.
By End-User: Academic
The Academic end-user segment is a crucial driver of platform adoption, fueled by the need to accelerate fundamental scientific breakthroughs and foster interdisciplinary collaboration. Unlike commercial pharmaceutical entities, academic institutions' demand is less focused on immediate return-on-investment and more on the utility of tools that can transcend disciplinary boundaries. Graph-Based Hypothesis Generation Platforms are particularly sought after, as they allow researchers to model the interconnections between disparate scientific fields (e.g., materials science, genomics, chemistry) within a unified knowledge graph. This capability directly increases the volume and novelty of research hypotheses generated, transforming the research process from a literature-driven effort to a data-driven one. Furthermore, the push for open science and reproducibility in US academic research drives demand for platforms that provide transparent, traceable, and shareable hypothesis generation methods, which is often facilitated by the deployment of Cloud-Based solutions that enable collaborative computing across geographically dispersed research teams.
________________________________________
Competitive Environment and Analysis
The US AI-Driven Hypothesis Generation competitive landscape is bifurcated between major technology firms offering generalized AI capabilities and specialized "TechBio" companies leveraging proprietary biological data sets and domain-specific algorithms. Competition is centered on the depth of proprietary data, the defensibility of the underlying ML models, and the verified progress of pipeline assets.
Recursion Pharmaceuticals
Recursion Pharmaceuticals is strategically positioned as a clinical-stage TechBio company that leverages a proprietary industrial-scale wet-lab—the "Recursion Operating System (OS)"—to generate one of the world's largest biological and chemical datasets. Its strategic advantage is the closed-loop feedback mechanism, where the proprietary data trains the Recursion OS, which then fuels the drug discovery pipeline. The company's focus is on decoding biology to identify potential first-in-class and best-in-class treatments for oncology and rare diseases, with verifiable pipeline assets in clinical and preclinical stages. A major strategic move was the August 2024 announcement of an acquisition of AI rival Exscientia, aiming to consolidate leading AI drug pioneers and accelerate the joint pipeline, signaling a drive toward vertical integration of AI-driven discovery and development.
Atomwise
Atomwise is positioned as a leader in applying deep convolutional neural networks for small molecule drug discovery. Its core product is the AtomNet® technology, a deep learning-based drug discovery engine that rapidly analyzes billions of chemical compounds to predict bioactivity and toxicity, which directly accelerates hit identification and lead optimization for partners. The company's strategic positioning relies on high-value, de-risked partnerships with major pharmaceutical companies. Its official publications highlight collaborations, such as the expanded AI partnership with Hansoh and a follow-on research agreement with Bayer, demonstrating the commercial validation of its AI-driven platform in unlocking challenging targets and increasing the potency and selectivity of compounds.
________________________________________
Recent Market Developments
- July 2025: HPE announced the successful completion of its acquisition of Juniper Networks, a leader in AI-native networks. This merger creates an industry-leading cloud-native and AI-driven IT portfolio with a full networking stack. The integration will accelerate customers' deployment and adoption of hybrid cloud and AI workloads, fundamentally bolstering the computational infrastructure that underlies scalable AI-Driven Hypothesis Generation solutions.
- August 2024: Recursion announced its acquisition of Exscientia, combining two major players in the AI-driven drug discovery space. This strategic merger was positioned to create a unified AI-first engine for drug discovery and development, aimed at accelerating the discovery of new medicines by consolidating complementary data sets and algorithmic expertise.
________________________________________
US AI-Driven Hypothesis Generation Market Segmentation
- BY SOFTWARE TYPE
- AI-Powered Literature Mining Tools
- Graph-Based Hypothesis Generation Platforms
- Domain-Specific Predictive Modeling Tools
- Multimodal AI Platforms
- Others
- BY APPLICATION AREA
- Drug Discovery & Life Sciences
- Healthcare & Diagnostics
- Materials & Chemical Research
- Financial & Business Analytics
- Academic
- BY DEPLOYMENT MODE
- Cloud-Based
- On-Premise