Report Overview
Cerebral Protection System Market Highlights
Cerebral Protection System Market Size:
The cerebral protection system market is projected to grow at a CAGR of 8.14% over the forecast period, increasing from US$1,033.241 million in 2025 to US$1,528.241 million by 2030.
The global cerebral protection system market is a fast-growing and dynamic business with applications in medical research, diagnostics, and medication development. Cerebral protection systems are also known as cell surface antigens or clusters of differentiation markers; they are proteins or molecules on the outer surface of the cells serving as identifying tags for those cells. These markers find widespread application in identifying, isolating, and characterizing many populations of cells helping better understand cell function and disease causation and which could help to identify targets for future therapies.
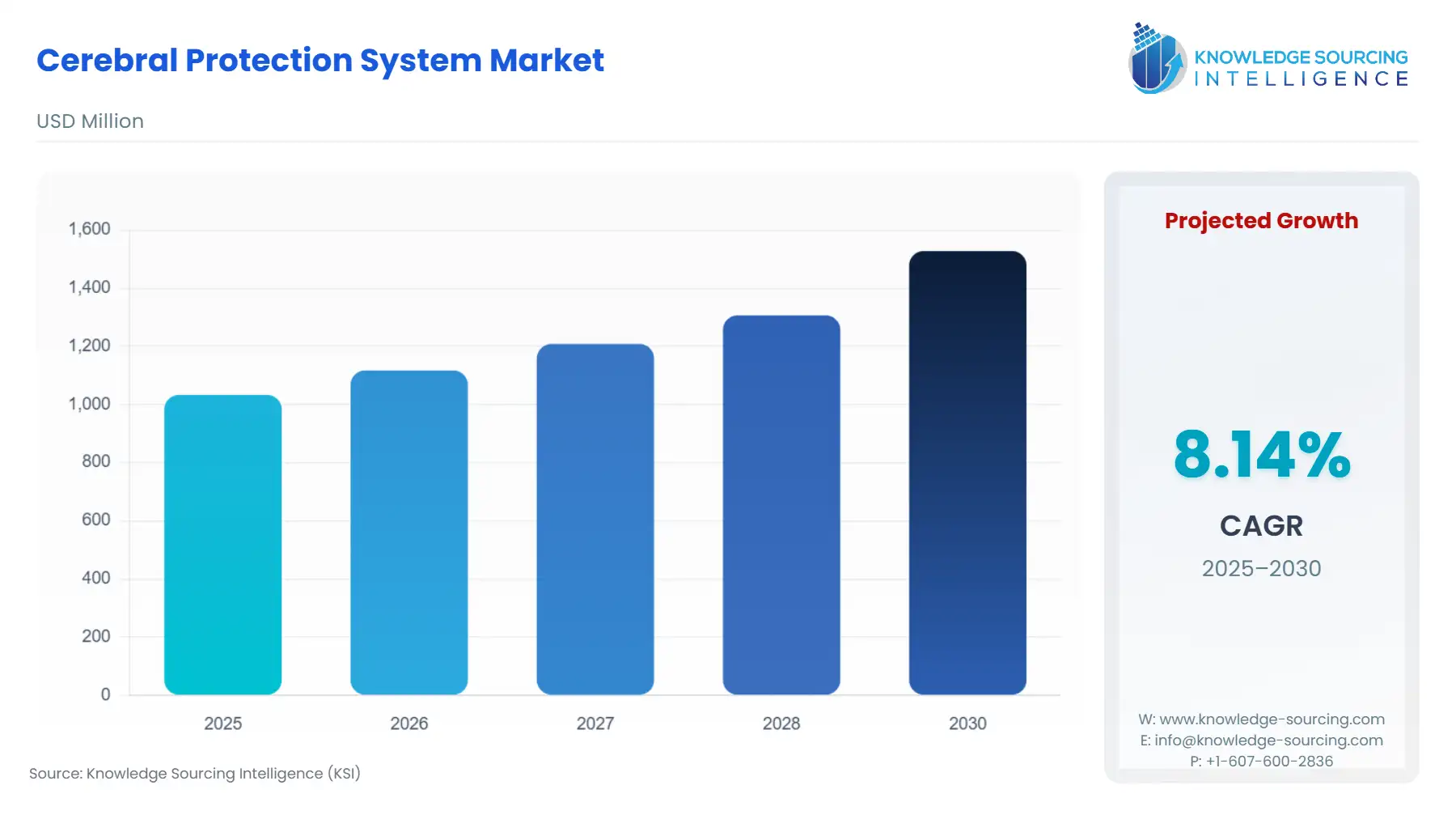
The growth in the market can be attributed to several factors, including the increasing prevalence of chronic diseases, the rising demand for custom therapies, advances in flow cytometry and other cell analysis methods, and an increase in spending on research and development activities.
Cerebral Protection System Market Growth Drivers:
- Rising cases of cardiovascular diseases are increasing the cerebral protection system market growth.
The demand for CPS or Cerebral Protection Systems has grown tremendously owing to the increasing incidence of cardiovascular diseases, predominantly aortic stenosis. Aortic stenosis is defined as the narrowing of the aortic valve, and the older the population gets, the more it is of global concern. Such an issue often requires surgical procedures such as Transcatheter Aortic Valve Replacement (TAVR), which are associated with the risk of embolism to the brain. Hence, there is a great demand for CPS to minimize these situations during such operations.
Moreover, as people grow older, the number of age-related cardiovascular diseases increases considerably. Most of the elderly would be perfect candidates for minimally invasive procedures including TAVR that present with quite several age-related complication concerns such as aortic stenosis. This has increased the incidence of demand for CPS, which is expected to keep patients safe while undergoing such procedures.
- Rising technological advancement is anticipated to increase the market demand.
Emerging technologies have ultimately made CPS more effective and efficient, as far as medical devices are concerned. Several devices such as SENTINEL Cerebral Protection System and Triguard 3 CEP Device Technology have been involved in capturing and removing embolic debris during specific procedures such as TAVR. That momentum has created more meaningful benefits for the patients in terms of health improvement and increased reliance on CPS in operative scenarios.
Moreover, surgery has become an increasingly popular option among patients and healthcare providers alike, thanks to its associated benefits: faster recovery times coupled with reduced risks. These minimally invasive procedures are gaining traction particularly, TAVR using CPS-which is even triggered for greater adoption. Increased inclination of patients towards minimally invasive treatment options will surge the demand for CPS.
- Increasing government initiatives is anticipated to increase the market demand.
Government initiatives and increased investments in the healthcare sector have acted as the primary growth engines for this industry. For instance, it has become mandatory for governments to invest increasingly in sophisticated medical technologies like CPSs now that cardiovascular diseases have become the leading global enemies. That is why the U.S. National Institutes of Health (NIH) has funded multiple studies on embolic protection devices in America to hasten innovation and create new CPS products for the marketplace. Within the framework of the largest EU research and innovation programme, Horizon Europe, projects are funded in the areas of medical technology and cardiovascular health care. Such incentives promote collaboration between research institutes and device manufacturers, facilitating the development of more efficient CPS solutions.
Cerebral Protection System Market Key Segments:
- By product, clarets sentinel system is anticipated to grow during the forecast period.
A Clarets sentinel system is an embolic protection device that operates on the principle of inertial separation to keep potentially dangerous objects from entering the bloodstream. The system may be incorporated with TAVI in providing Cerebral Embolic Protection (CEP). The Triguard Device from Keystone Hearts is a protective device applied during cardiopulmonary bypass to protect the brain. It also reduces the risk of brain damage and cerebral bleeding in the surgery patients. An Umbrella Embolic Deflector is a basic umbrella-type device that diverts the blood with a catheter to prevent embolism. It keeps the air inside to maintain its shape and prevents emboli from getting through the cerebral veins.
- By application, the hospital segment is anticipated to grow during the forecast period.
The demand for cerebral protection systems in hospitals is gradually ramping up because of their usefulness in avoiding complications associated with some cardiovascular procedures affecting the heart and brain. It primarily protects the patient from embolic events like stroke during transcatheter aortic valve implantation (TAVI) or coronary artery bypass grafting (CABG). With increased awareness and emphasis on patient safety, hospitals integrate CPS as part of the standard of care with high-risk cardiovascular procedures. The purpose is to reduce the risk of strokes and improve long-term outcomes in patients, making it a very important tool in the surgical arsenal.
Cerebral Protection System Market Geographical Outlook:
- Asia Pacific is witnessing exponential growth during the forecast period.
Asia Pacific has shown rapid growth in cerebral protection systems in the global market. Various cardiovascular disorders such as aortic stenosis, and coronary artery disease among others continue to contribute to increasing intervention procedures within the Asia Pacific region. The procedures include Transcatheter Aortic Valve Implantation (TAVI), frequently associated with the usage of CPS to reduce the risk of embolic events during surgery. Advanced medical technologies are increasingly being implemented in hospitals throughout the Asia Pacific to improve patient outcomes. Integration of CPS into cardiovascular therapies reveals a commitment to improving patient safety and reducing the incidence of stroke and other brain sequelae associated with these therapies.
Cerebral Protection System Market Key Developments:
- In November 2024, A comprehensive assessment of the current state of cerebral embolic protection (CEP) devices used in TAVR treatments was published. The paper examines significant clinical trials and current advances from 2016 to the present, focusing on the evolution and usefulness of CEP devices in lowering periprocedural stroke risks.
Cerebral Protection System Market Scope:
| Report Metric | Details |
|---|---|
| Study Period | 2021 to 2031 |
| Historical Data | 2021 to 2024 |
| Base Year | 2025 |
| Forecast Period | 2026 – 2031 |
| Report Metric | Details |
| Cerebral Protection System Market Size in 2025 | US$1,033.241 million |
| Cerebral Protection System Market Size in 2030 | US$1,528.241 million |
| Growth Rate | CAGR of 8.14% |
| Study Period | 2020 to 2030 |
| Historical Data | 2020 to 2023 |
| Base Year | 2024 |
| Forecast Period | 2025 – 2030 |
| Forecast Unit (Value) | USD Million |
| Segmentation |
|
| Geographical Segmentation | North America, South America, Europe, Middle East and Africa, Asia Pacific |
| List of Major Companies in the Cerebral Protection System Market | |
| Customization Scope | Free report customization with purchase |
The Cerebral protection system market is analyzed into the following segments:
- By Type
- By Product
- By Application
- Hospitals
- Homes
- Others
- By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- Germany
- UK
- France
- Spain
- Others
- Middle East and Africa
- UAE
- Israel
- Others
- Asia Pacific
- China
- Japan
- South Korea
- India
- Indonesia
- Taiwan
- Thailand
- Others
- North America