Report Overview
Extracorporeal Membrane Oxygenation Machine Highlights
Extracorporeal Membrane Oxygenation Machine Market Size:
The extracorporeal membrane oxygenation machine market, with a 5.25% CAGR, is anticipated to reach USD 713.548 million in 2031 from USD 524.986 million in 2025.
The Extracorporeal Membrane Oxygenation (ECMO) Machine market is characterized by its vital role in providing prolonged cardiac and respiratory support to critically ill patients whose native organs have failed. This advanced life support technology, which includes a circuit of a pump, an oxygenator, and cannulas, temporarily assumes the function of the heart and/or lungs, allowing the affected organs to rest and potentially recover. Originally developed for neonatal respiratory failure, the application scope has broadened significantly to include refractory cardiogenic shock, severe ARDS, and extracorporeal cardiopulmonary resuscitation (ECPR) in adult and pediatric populations. The market's operational landscape is defined by the necessity for highly specialized clinical expertise, substantial capital investment, and stringent regulatory oversight, positioning it firmly within the tertiary care and academic medical center environments globally.
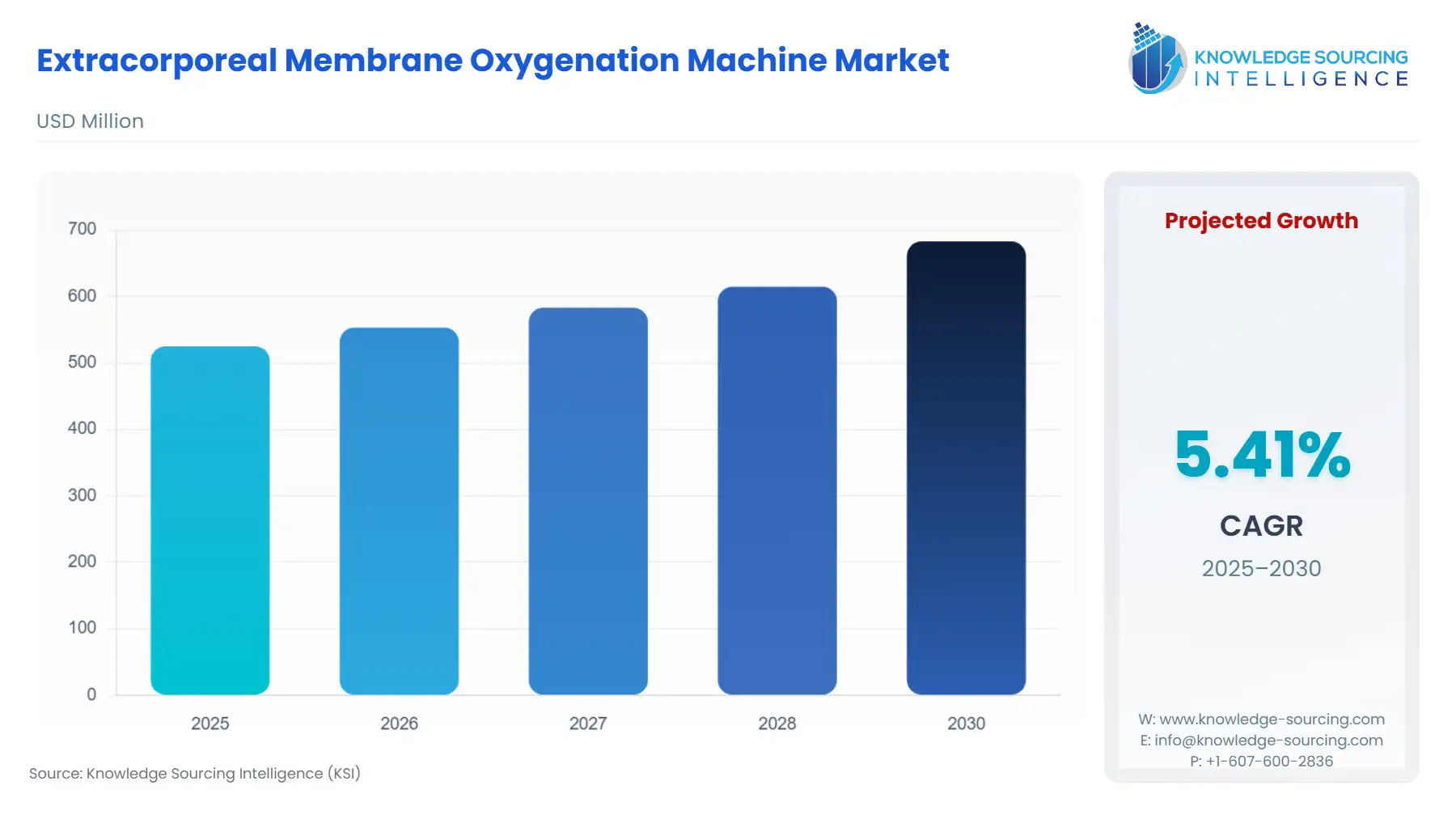
Extracorporeal Membrane Oxygenation Machine Market Analysis:
Growth Drivers:
The rising incidence of chronic obstructive pulmonary disease (COPD) and acute respiratory distress syndrome (ARDS) significantly increases the patient pool requiring advanced respiratory support. As conventional mechanical ventilation can induce further lung injury, the utilization of Veno-Venous (VV) ECMO offers a lung-rest strategy, directly creating demand for these specialized circuits and the main ECMO unit. Concurrently, the increasing prevalence of cardiovascular diseases, such as heart failure and refractory cardiac arrest, necessitates Veno-Arterial (VA) ECMO for temporary cardiopulmonary bypass. The imperative to improve survival rates in these conditions acts as a direct catalyst for hospital systems to expand and maintain their ECMO capacity, driving demand for both new machine installations and a consistent supply of disposable components.
Challenges and Opportunities:
A primary challenge is the exorbitant total cost of care, encompassing the machine, single-use circuits, and the lengthy, high-acuity intensive care unit (ICU) stay. This constraint directly suppresses demand in budget-sensitive healthcare systems and limits accessibility, thereby impacting total procedure volume. Conversely, a significant opportunity lies in the development of portable and miniaturized ECMO systems. These advancements enhance the ability to safely transport critically ill patients, expanding the clinical application from in-ICU rescue therapy to pre-hospital or inter-hospital transfer support. This technological shift broadens the demand profile from fixed hospital units to include emergency medical services and specialized transport teams, generating a new segment of demand.
Raw Material and Pricing Analysis:
The Extracorporeal Membrane Oxygenation Machine is a physical product composed of durable hardware and single-use consumables. The key consumable components, particularly the oxygenator, rely heavily on polymer sciences, specifically polymethylpentene (PMP) for the hollow-fiber membrane, and biocompatible coatings, such as heparin, for the entire blood-contacting circuit. Price stability in the highly specialized polymer and chemical coating market is crucial, as any volatility in the cost of these medical-grade raw materials directly influences the pricing of the disposable oxygenator and cannula sets, which represent the consistent, high-volume revenue stream for manufacturers. As component innovation, such as improved thrombogenic resistance, increases, the premium pricing for these advanced disposable circuits is justified by the clinical imperative for improved patient safety and prolonged run times.
Supply Chain Analysis:
The global supply chain for ECMO systems is centralized, relying on specialized manufacturing hubs, predominantly in North America and Europe, where the dominant market players are headquartered. The primary logistical complexity stems from the need for precision manufacturing and sterilization of the disposable circuits, which are composed of multiple specialized components, including the oxygenator, centrifugal pump head, and cannulas. The system exhibits a dependency on high-purity medical-grade polymers and specialized anti-thrombotic coatings, which can introduce vulnerability to global chemical supply disruptions. The distribution model is highly regulated, necessitating rigorous temperature and environmental controls to deliver sterile, ready-to-use disposable components directly to tertiary care hospital ICUs and operating rooms.
Government Regulations:
Jurisdiction | Key Regulation / Agency | Market Impact Analysis |
|---|---|---|
United States | FDA (Food and Drug Administration) | Mandates Pre-Market Approval (PMA) or 510(k) clearance for ECMO devices. This stringent regulatory path dictates the pace of new product introduction, ensuring safety and efficacy but creating a high barrier to entry and a lengthy product development cycle, which can slow the adoption of new technologies. |
Europe | European Medicines Agency (EMA) / CE Mark | Requires adherence to the Medical Device Regulation (MDR) for market access. The focus on comprehensive clinical data and post-market surveillance provides manufacturers with a standardized, though complex, path to market, supporting competitive product launches across the EU, thereby potentially increasing demand through broader product availability. |
China | NMPA (National Medical Products Administration) | Governs the registration and approval of imported and domestically manufactured devices. The strict import registration process and emphasis on local clinical trials create a significant market barrier for international players, but simultaneously catalyze domestic manufacturing and R&D investment, fostering localized supply chains and increased demand for NMPA-approved devices. |
Extracorporeal Membrane Oxygenation Machine Market Segment Analysis:
By Modality Outlook: Veno-Venous (VV) ECMO The Veno-Venous (VV) ECMO segment focuses exclusively on respiratory support, bypassing the lungs while allowing the heart to continue functioning. The core growth driver for VV ECMO is the increasing worldwide prevalence of severe, refractory ARDS, notably highlighted by the global health crisis but also driven by conditions like severe pneumonia, trauma, and inhalation injury. The clinical rationale is to apply a "lung rest" strategy, minimizing the ventilator-induced lung injury (VILI) associated with high-pressure conventional mechanical ventilation. The growing evidence from large-scale clinical trials supporting improved survival outcomes when ECMO is utilized for severe ARDS, compared to conventional management, directly fuels demand. This has prompted academic medical centers and large hospital networks to invest in dedicated ECMO centers of excellence, requiring not only the main ECMO console but a continuous supply of specialized VV cannulas and high-efficiency oxygenators optimized for gas exchange. The necessity is further concentrated in adult critical care, which accounts for the largest proportion of VV ECMO usage.
By End-User: Hospitals Hospitals, specifically tertiary care and academic medical centers, represent the dominant and core end-user segment for the Extracorporeal Membrane Oxygenation Machine Market. This segment's growth is driven by the concentration of complex surgical procedures and high-acuity critical care management in these institutions. ECMO therapy necessitates an intricate, multidisciplinary team—including cardiothoracic surgeons, perfusionists, intensivists, and specialized nursing staff—which is typically only available in a hospital setting with substantial infrastructure. The application of ECMO in emergent settings like Extracorporeal Cardiopulmonary Resuscitation (ECPR) and as a bridge-to-transplant or bridge-to-decision for patients in profound cardiac or respiratory failure locks the demand exclusively into these high-capability hospitals. Furthermore, governmental initiatives and regional critical care networks increasingly designate these institutions as regional ECMO referral centers, which mandate continuous investment in both new, portable ECMO units for transport and regular replacement of high-throughput disposable components.
Extracorporeal Membrane Oxygenation Machine Market Geographical Analysis:
United States Market Analysis
The US market is characterized by rapid technological adoption and a high concentration of sophisticated, well-funded tertiary care centers. The primary growth driver is a favorable reimbursement landscape, coupled with the rising number of patients suffering from end-stage cardiopulmonary diseases, including a high incidence of cardiovascular disease. Continuous investment by major market players in R&D, often in collaboration with leading US academic centers, ensures that new, miniaturized, and integrated ECMO systems are commercialized quickly. This early adoption solidifies the US market's leadership and drives per-capita utilization rates higher than in most other regions.
Brazil Market Analysis
The Brazilian market faces a dual structure: well-equipped private hospitals in major metropolitan areas drive sophisticated ECMO requirements, while the public health system (SUS) struggles with resource constraints. Local demand is propelled by the large population and a significant burden of infectious respiratory diseases, but the high capital cost of the ECMO hardware and the complexity of the disposable circuit supply chain present significant financial barriers. This creates a highly segmented demand, with growth concentrated in high-end private healthcare, where the cost of the intervention can be reliably absorbed.
Germany Market Analysis
Germany's market benefits from a robust and decentralized healthcare infrastructure, high healthcare spending, and a strong culture of clinical excellence. Local demand is spurred by well-established standards of critical care and proactive government initiatives to equip hospitals with advanced life support technologies. German hospitals are often early and comprehensive adopters of the latest ECMO technologies, including advanced oxygenator designs and integrated transport systems, cementing Europe's leadership in clinical utilization and consistent consumable demand.
Saudi Arabia Market Analysis
The ECMO market in Saudi Arabia is largely driven by substantial government investments aimed at modernizing healthcare infrastructure, particularly in specialized critical care and cardiac surgery centers of excellence. Its necessity is fundamentally governmental-driven, focusing on acquiring world-class medical technology to address the regional rise in lifestyle-related cardiac diseases. Local factors, such as the need for comprehensive clinician training programs, are crucial, as is the reliance on highly efficient, centralized logistics to manage the imported supply of disposable ECMO circuits across the Kingdom.
China Market Analysis
China represents a rapidly expanding market, characterized by immense patient volume and an accelerating rate of infrastructure development. The segment's growth is propelled by the government's push to expand access to high-quality critical care beyond first-tier cities, alongside the vast burden of chronic respiratory and cardiovascular diseases. The primary local dynamic is the increasing preference for, and growth of, domestically manufactured ECMO machines and components, spurred by National Medical Products Administration (NMPA) approval processes and a strategic imperative to reduce reliance on imported foreign devices.
Extracorporeal Membrane Oxygenation Machine Market Competitive Environment and Analysis:
The Extracorporeal Membrane Oxygenation Machine market is dominated by a few multinational corporations that leverage long-established relationships within the cardiopulmonary and perfusion technology segments. Competition centers on technological differentiation, particularly in biocompatibility, system portability, and integration of advanced monitoring features, alongside the cost and reliability of the disposable oxygenator and pump circuits.
Medtronic plc Medtronic, a global leader in medical technology, maintains a strong competitive position in the ECMO market by integrating its cardiopulmonary support offerings. The company strategically leverages its extensive global distribution network and expertise in perfusion systems to drive the adoption of its ECMO portfolio. A key product is the VitalFlow ECMO system, which features a simplified, fully integrated design intended to bridge the gap between bedside care and intra-hospital transport. Medtronic strengthened this position through the acquisition of MC3 Cardiopulmonary in March 2024, aiming to offer a comprehensive, one-system ECMO solution across key markets.
LivaNova LivaNova focuses its strategy on advanced circulatory support and cardiopulmonary products. The company's competitive edge is built on providing comprehensive systems, including the LifeSPARC System, which is intended for temporary cardiopulmonary bypass and extracorporeal life support (ECLS). The LifeSPARC system is designed around a centrifugal blood pump, aiming to provide a user-friendly and effective solution for adult patients with acute respiratory or cardiac failure. LivaNova employs a strategic focus on expanding indications for its circulatory support technology, aiming to enhance its market share through technological innovation in the cardiac and respiratory support domain.
Getinge AB Getinge, a major player in products and systems for surgery, intensive care, and cardiovascular procedures, positions its ECMO offerings under its flagship perfusion and life support portfolio. The company's focus is on providing reliable, high-performance systems and consumables to critical care environments. Getinge's offerings, which include the Cardiohelp System, emphasize patient safety, transport capability, and ease of use in diverse clinical settings, maintaining a competitive position through a robust product range for both cardiac surgery and critical care applications.
Extracorporeal Membrane Oxygenation Machine Market Recent Developments:
September 2024: Medtronic initiated the rollout of its VitalFlow ECMO one-system solution in the U.S. following its acquisition of MC3 Cardiopulmonary in March 2024. The introduction of the VitalFlow system, which includes the Medtronic Nautilus oxygenator design, directly increases the demand for their new, integrated consumables portfolio. This product launch positions Medtronic to capture a larger market share for portable and easily transportable ECMO support devices in the critical care environment.
March 2024: Medtronic announced the acquisition of MC3 Cardiopulmonary, a company specializing in advanced technologies for extracorporeal life support. This merger and acquisition event was explicitly undertaken to consolidate and expand Medtronic's cardiac surgery portfolio, immediately enhancing its capacity to deliver the next generation of integrated ECMO solutions. The acquisition strengthens Medtronic's competitive position and increases its production capacity and product range to meet the rising global demand for comprehensive ECMO systems.
List of Top Extracorporeal Membrane Oxygenation Machine Companies:
Medtronic plc
LivaNova
Terumo Cardiovascular Systems Corporation
Nipro Medical Corporation
Microport Scientific Corporation
Segmentation
By Product
ECMO Machine
Software
By Component
Pumps
Oxygenators
Cannul
Accessories
By Modality Outlook
Veno-Venous
Veno-Arterials
Arterio-Venous
By Geography
North America
USA
Canada
Mexico
South America
Brazil
Argentina
Others
Europe
Germany
France
United Kingdom
Spain
Others
Middle East and Africa
Saudi Arabia
UAE
Others
Asia Pacific
China
India
Japan
South Korea
Indonesia
Thailand
Others