Report Overview
AI-powered Clinical Trial Management Highlights
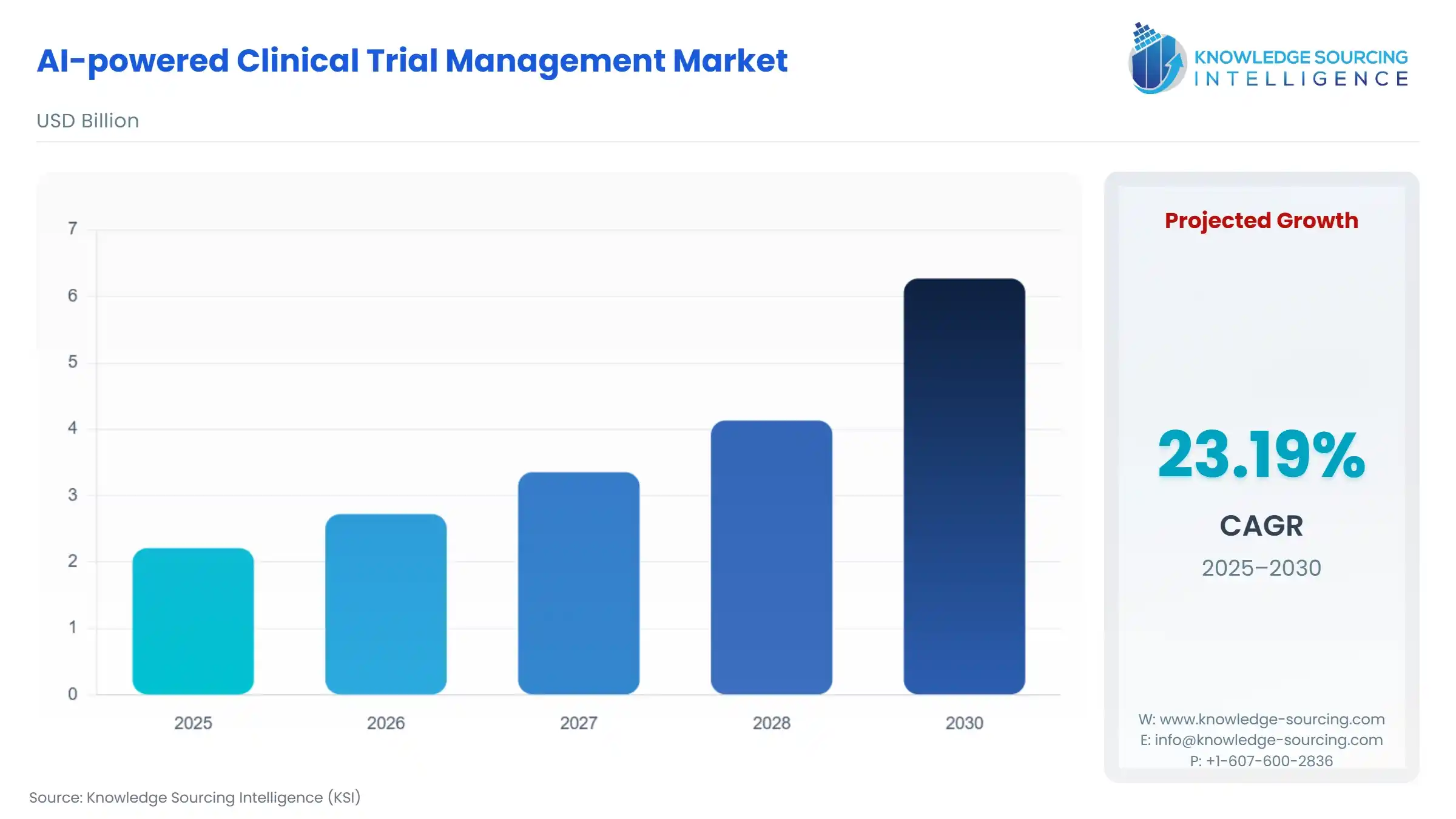
AI-Powered Clinical Trial Management Market Size:
The AI-Powered Clinical Trial Management Market is expected to grow at a CAGR of 23.19%, reaching a market size of US$6.270 billion in 2030 from US$2.210 billion in 2025.
The market for using AI in healthcare has experienced a significant boom with a rise in the adoption of AI by the major players. The AI-driven clinical trial management market speeds up and improves various stages of clinical trials via patient recruitment, data analysis, and protocol optimization. AI makes drug development more efficient, cheaper, and quicker by automating repetitive tasks and providing predictive insights. This revolutionary technology can change the entire process of medical research, enabling more accurate, swift decisions. Due to increasing uptake by pharmaceutical companies, research institutions, and healthcare providers of AI-based solutions, this market is expected to grow substantially, transforming its landscape and eventually improving patient care.
The market for providers of AI-based clinical trial solutions is being driven by the growing adoption of AI-based platforms to enhance the efficiency and effectiveness of trials at different stages. One factor driving the market expansion is the public and private sectors' supportive initiatives for various therapeutic areas. Additionally, the market is growing due to the increased awareness and various uses of AI in clinical trials, including better patient selection, site selection, drug trial design, patient monitoring, and more.
AI-Powered Clinical Trial Management Market Drivers:
- Increased demand for drug trials is anticipated to increase the market demand
As a result of the growing application of artificial intelligence to drug trials and the availability of different AI solutions designed specifically for pharmaceutical research, such as medication compliance, trial design, selection of investigators or sites, patient monitoring, etc., the area offering AI-based clinical trial solutions is widening. Enrollment and patient eligibility are two crucial processes for the overall success of the medication trial. According to studies, 30% of medication trials end early because of patient recruitment failure, and 85% of trials are postponed during patient recruitment. Platforms with an AI component are helping to lower this barrier. In turn, the market for AI-based clinical trial solution providers is driven by several researchers utilizing AI in drug trials.
- Increased research & development are anticipated to drive the market growth
Various R&D initiatives have been carried out by multiple organizations in the public and private sectors over recent years to support several therapeutic areas. This characteristic is predicted to provide new chances for expansion in the market of AI-based clinical trials in the years ahead. Numerous companies are investing heavily in R&D globally regarding AI-based clinical trials. The relocation is assisting them with the tasks associated with new product development and, ultimately, the introduction of new products. According to this trend, the market for providers of AI-based clinical studies will be very optimistic in the future.
- Growing use of AI-based platforms
The market for AI-based clinical trials, which aim to improve the effectiveness and efficiency of trials at different stages, is driven by the increasing use of AI-based platforms. The government and private sectors' support for different therapeutic areas is one of the additional factors driving the market expansion. Growing knowledge of AI's uses in clinical trials—including drug trial design, improved patient selection, site selection, patient monitoring, etc.—is also helping the market.
AI has the potential to reduce bias in medical data as well. To counteract bias in drug studies, for example, Genentech and Stanford University collaborated to develop an open-source AI system. The market is expanding because well-known pharmaceutical companies extensively use AI-based technologies in clinical trials. This is a result of the trend toward technology-based methods and away from antiquated practices.
- Hyper-personalized medicine and trial design
AI can analyze patient information, such as genes, way of life, and health background, to make individualized treatment plans. This method increases the effectiveness of treatment by considering each individual's specific response to therapy.
Further, AI facilitates classifying patients according to characteristics, allowing for more precise and targeted recruitment for clinical trials. This shortens trial runs and increases the likelihood that patient groups will benefit significantly from treatment. Hyper-personalized medicine powered by AI enhances patient outcomes and satisfaction. Such a strategy ought to enhance the validity of research findings because it may also reduce the number of clinical trial dropouts, precluding participant re-recruitment and, thus, sustaining test subjects for a longer time.
AI-Powered Clinical Trial Management Market Restraints:
- Standardization of the AI model is anticipated to impede market growth
The extensive amount of health data, such as that on patients, imaging information, and electronic health records, requires customized AI models. However, the need for defined protocols and frameworks makes it difficult to create broadly applicable AI models in the context of clinical trials.
Further, when healthcare organizations use different data formats, collection methods, and standards, it can be more difficult to successfully integrate artificial intelligence solutions in different scenarios. Standardizing AI models for clinical trials is also made more difficult by local ethical and legal constraints.
AI-Powered Clinical Trial Management Market Geographical Outlook:
- North America is witnessing exponential growth during the forecast period
Regarding technological progress and innovation concerning artificial intelligence, North America, particularly the United States, has been at the forefront of the world. Numerous premier information technology organizations, learning institutions, and start-ups focused on preparing state-of-the-art AI answers for various industries, such as medical research or the health sector, are found in this region. A lot of funds and materials have been allocated to AI companies as well as research projects. More capital ventures, public sectors, and individual financiers have expressed their interest in creating and utilizing AI technologies in healthcare services, including clinical trials.
AI-powered Clinical Trial Management Market Key Launches:
- In September 2023, Clarivate Plc, a global leader in providing people and organizations with trustworthy intelligence to transform the world, announced the establishment of an Academia & Government Innovation Incubator. This will accelerate its strategy for leveraging AI, encouraging creativity, and introducing innovative products for its user base and academic clients.
- In July 2023, Insilico Medicine raised the bar in artificial intelligence drug research by moving the first drug discovered and produced by generative AI into Phase II clinical trials involving humans. INS018_055, the main program, is a pan-fibrotic inhibitor that might be the first of its type. The success of Insilico's moonshot drug demonstrates the viability of Pharma. AI, the company's end-to-end AI drug development platform.
List of Top AI-Powered Clinical Trial Management Companies:
- Medidata Solutions
- Oracle Corporation
- IBM Corporation
- Veeva Systems
- Clinerion
AI-Powered Clinical Trial Management Market Scope:
| Report Metric | Details |
|---|---|
| Total Market Size in 2026 | USD 2.210 billion |
| Total Market Size in 2031 | USD 6.270 billion |
| Growth Rate | 23.19% |
| Study Period | 2021 to 2031 |
| Historical Data | 2021 to 2024 |
| Base Year | 2025 |
| Forecast Period | 2026 – 2031 |
| Segmentation | Type of AI solution, Function, End-User, Geography |
| Geographical Segmentation | North America, South America, Europe, Middle East and Africa, Asia Pacific |
| Companies |
|
AI-Powered Clinical Trial Management Market Segmentation:
- By Type of AI solution
- Clinical Trial Planning and Design
- Patient Recruitment and Enrolment
- Data Management and Analysis
- Monitoring And Oversight
- Safety And Pharmacovigilance
- By Function
- Predictive Analytics
- Natural Language Processing (NLP)
- Machine Learning
- Robotic Process Automation (RPA)
- Image And Signal Processing
- By End-User
- Pharmaceutical Companies
- Contract Research Organizations (Cros)
- Academic And Research Institutions
- Biotechnology Companies
- Medical Device Manufacturers
- By Geography
- North America
- United States
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- United Kingdom
- Germany
- France
- Italy
- Spain
- Others
- Middle East and Africa
- Saudi Arabia
- UAE
- Others
- Asia Pacific
- Japan
- China
- India
- South Korea
- Taiwan
- Thailand
- Indonesia
- Others
- North America