Report Overview
Central Precocious Puberty Treatment Market Size
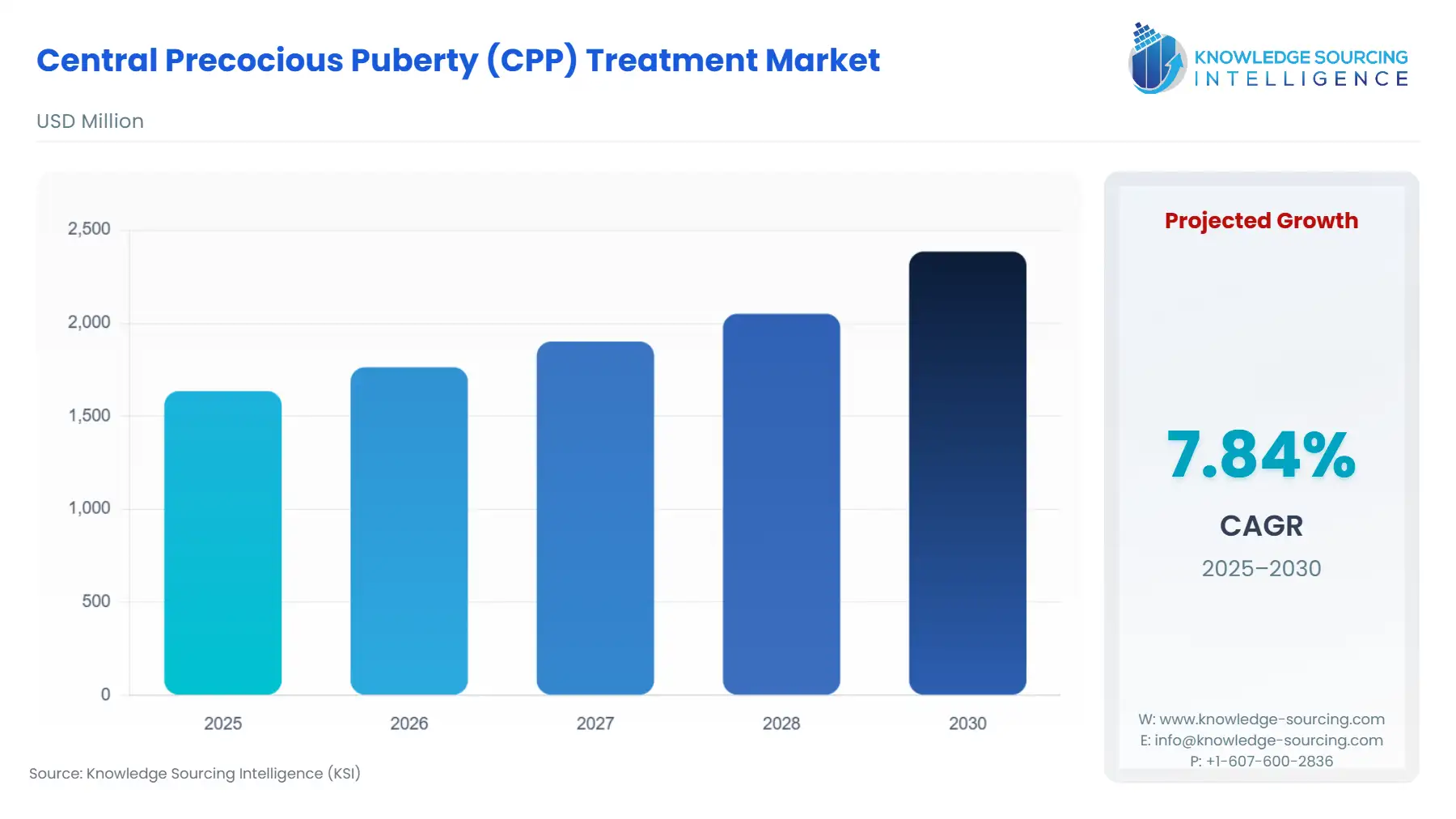
The Central Precocious Puberty (CPP) treatment market is expected to grow at a CAGR of 7.84%, reaching a market size of US$2,384.872 million in 2030 from US$1,634.872 million in 2025.
Central precocious puberty (CPP) treatment consists of medicinal therapies aimed at managing and postponing the precocious initiation of puberty caused by premature activation of the hypothalamic-pituitary-gonadal (HPG) axis. The precocious sexual maturation has been defined as the appearance of secondary sex characteristics before eight in girls and before nine in boys. It is to harness the gonadotropin-releasing hormone (GnRH) agonists, those which prevent sex hormone initiation as well as decelerate or stop the progress of puberty.
Moreover, the treatment of central precocious puberty (CPP) is surgical or medicinal. Therapies are administered to postpone the maturation of children, while an operation can be considered in a few cases. Blood tests, MRIs, CT scans, and X-rays help determine the need for treatment. The treatment is available for boys and girls and is primarily aimed at hospitals, specialized clinics, homecare setups, as well as other healthcare providers.
Central Precocious Puberty Treatment Market Growth Drivers:
- Increasing prevalence of hormonal problems is contributing to the Central Precocious Puberty (CPP) treatment market expansion.
It is anticipated that the increasing prevalence of hormonal disorders will contribute considerably to the advancement of the CPP therapy market in subsequent years. Disorders of hormonal causes are diverse medical conditions resulting from some hormonal discrepancies in the human body. Hormonal abnormalities have an increasing trend influenced by a modern lifestyle in addition to exposure from the immediate environment and genetic or epigenetic factors. The primary objective of a CPP treatment is to control the early-onset of puberty, generally brought about by some hormonal imbalance, by suppressing the hormonal signals that are responsible for the onset of puberty. Hence, the rising incidence of hormonal abnormalities is a major driver for the CPP treatment market.
- Use of approved therapies is anticipated to increase the market demand.
The demand for Triptodor, Lupron Depot-Ped, and Supprelin LA (histrelin acetate) for the treatment of precocious puberty is expected to drive the growth of the market. These have been the gonadotropin-releasing hormone agonists approved for therapy in central precocious puberty. These agonists extend inhibition of gonadotropin secretion as clinically required, which should further boost growth. In addition to these, triptorelin, as a GnRH agonist, inhibits significantly the pituitary-gonadal axis and pubertal development in early publicized children, which would reflect on growth during the forecast period in the precocious puberty treatment market. Other factors expected to spur market growth include the rising incidence of hormonal disorders while increased rates of obesity are anticipated to contribute to growth during the forecast period.
- Rising healthcare infrastructure is also increasing the market demand.
One of the significant factors driving the growth rate for early commencement of puberty treatment market includes increased healthcare expenditure that improves the infrastructure. The market is also predicted to rise due to increased initiatives by government and private groups to create awareness concerning abnormalities related to early onset of puberty. The market for precocious puberty treatment would also be propelled by increased disposable income and growing needs for treating diseases with specific treatments. Similarly, increased radiation or chemotherapy of cancer patients and lifestyle habits of individuals are also going to increase the market in the forecast period.
- Rising disposable income & technological advancements are anticipated to increase the market demand.
The growing disposable income mostly stimulates the growth of the sugar-free beverages market. Due to increased purchasing power, customers tend to spend more on premium, healthier beverages. Innovative technology on sweeteners keeps sugar-free beverages at an advantageous position in availability to consumers. Natural and low-calorie sweeteners are expected to result in growth in the market as advancement continues. Moreover, likely active forces for the sugar-free beverage industry are rising regulatory actions and policies promoting reduced sugar consumption.
Central Precocious Puberty Treatment Market Key Segments:
- By drug type, Leuprorelin is anticipated to grow during the forecast period.
Leuprorelin, a gonadotropin-releasing hormone (GnRH) agonist, has proven itself to be a potent agent in the management of Central Precocious Puberty (CPP), a condition in which early puberty occurs in children. With the advancement of pediatric endocrinology, increased awareness of the condition, and improvements in diagnosis, demand for leuprorelin to treat CPP has continued to emerge.
Moreover, the increased incidence of CPP worldwide also depends on many factors like genetic predisposition, environmental effects, and increased awareness about the condition. Since leuprorelin inhibits sexual precocity by suppressing the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), it has become an important intervention for these children. Hence, the rise in demand for the drug is from the medical providers and parents who want to manage the disease.
Central Precocious Puberty Treatment Market Geographical Outlook:
- Asia Pacific is witnessing exponential growth during the forecast period.
The demand for Central Precocious Puberty (CPP) therapy in the Asia-Pacific region is at an impressive level that is expected to witness more growth in the awareness, health systems, and prevalence of the disorder. Increased development of diagnostic skills, increasing awareness among parents and health care professionals, and plausible environmental effects have come to cause a rise in the area. Streamlining of life and urbanization has changed diets and exposure to possible endocrine-disrupting substances that often attach to early puberty. More and more children are being diagnosed and treated for CPP due to awareness campaigns and educational programs that sensitize people about the benefits of early diagnosis and treatment.
Central Precocious Puberty Treatment Market Key Developments:
- In September 2024, Debiopharm launched Phase III clinical research called LIBELULA to investigate Debio 4326, a 12-month triptorelin formulation for children with CPP. This extended-release formulation promises to reduce the treatment burden by requiring only one injection per year, as opposed to the more frequent dosage schedules now in use. The first patients have been dosed, marking an important step in the development of this novel medicine.
Central Precocious Puberty Treatment Market Scope:
| Report Metric | Details |
|---|---|
| Study Period | 2021 to 2031 |
| Historical Data | 2021 to 2024 |
| Base Year | 2025 |
| Forecast Period | 2026 – 2031 |
| Report Metric | Details |
| Central Precocious Puberty Treatment Market Size in 2025 | US$942.165 million |
| Central Precocious Puberty Treatment Market Size in 2030 | US$2,384.872 million |
| Growth Rate | CAGR of 7.84% |
| Study Period | 2020 to 2030 |
| Historical Data | 2020 to 2023 |
| Base Year | 2024 |
| Forecast Period | 2025 – 2030 |
| Forecast Unit (Value) | USD Million |
| Segmentation |
|
| Geographical Segmentation | North America, South America, Europe, Middle East and Africa, Asia Pacific |
| List of Major Companies in the Central Precocious Puberty Treatment Market |
|
| Customization Scope | Free report customization with purchase |